Vulvar cancer in East Arnhem: 2007-2017
1. Background
In response to reports from outreach gynaecologists of excess cases of vulvar cancer and its precursor lesion, vulvar intraepithelial neoplasia (VIN), in the East Arnhem (EA) health district, investigations were undertaken from 2007. Funding was acquired from the National Health and Medical Research Council (NHMRC) for three projects investigating the epidemiological features and potential causes of the cluster (2007-2010; 2011-2013; 2014-2017).
Vulvar cancer is a relatively rare malignancy and has consequently not been well studied. Squamous cell carcinoma (VSCC) comprises the majority (>90%) of vulvar cancers.1 Mortality rates have remained steady, or increased in some regions, and patients suffer from associated morbidities that reduce quality of life.2-4
There are two distinct aetiological pathways for VSCC, resulting in separate forms of the disease.1,5,6 One is associated with infection with human papillomavirus (HPV), usually affects younger women, arises from vulvar high-grade squamous intraepithelial lesions (HSIL) (formerly usual type VIN (uVIN))7 and results in basaloid or warty carcinoma. The other variant is HPV-independent, usually affects postmenopausal women, is associated with differentiated VIN (dVIN) arising in an area affected by vulvar dermatoses such as lichen sclerosus, and leads to keratinizing VSCC.
Treatment for VSCC and VIN has evolved only marginally and continues to be associated with substantial physical morbidity and psychosexual dysfunction.8,9 Vulvar neoplasms are largely treated through surgery, irrespective of disease sub-type. A trend towards less radical surgery and an increase in the use of sentinel lymph node biopsies has gone some way towards reducing morbidity for patients with localized disease without affecting survival.8,10
2. Epidemiology and Confirmation of a Cluster
An initial epidemiological study was undertaken in 2007-08 in order to describe the epidemiological feature of this possible cancer cluster. NT-resident women with a confirmed histological diagnosis of vulvar cancer or VIN between 1 January 1996 and 31 December 2005 were identified. Seventy-one women were identified (32 vulvar cancer and 39 VIN), of whom the majority were Indigenous, aged less than 50 years and living in remote communities in the EA district.11
For the period 1996-2005, the age-adjusted incidence rate of vulvar cancer in EA Indigenous women aged 0–49 years was 31.1 per 100,000 (95% CI 13.1–49.1), over 50 times higher than the national Australian rate (0.4 per 100,000, 95% CI 0.4–0.5) for the same age-group (Figure 1).11
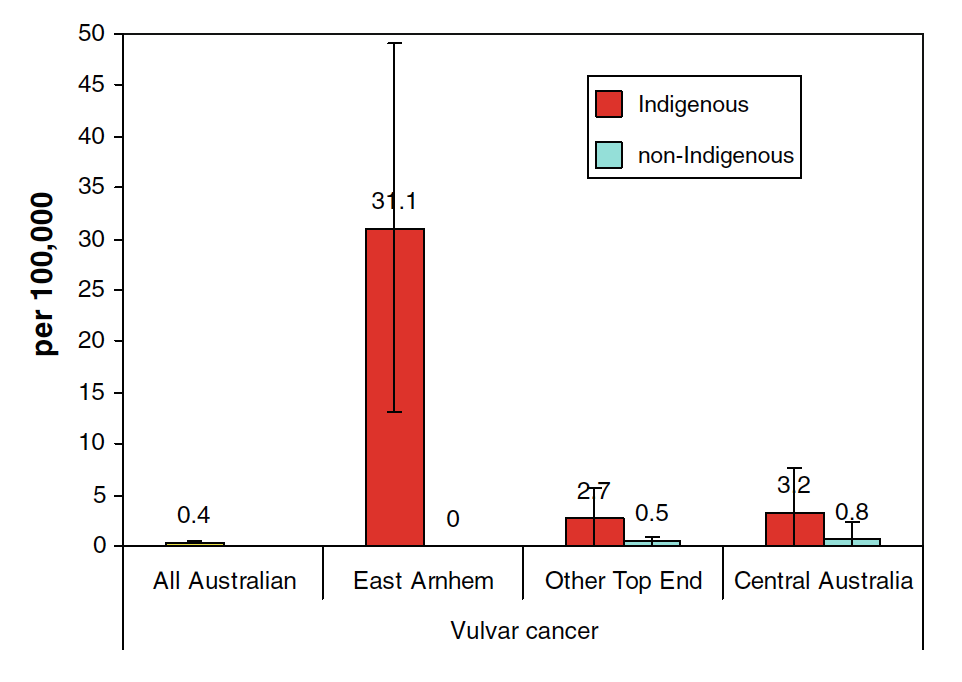
Figure 1 Age-adjusted incidence rate of vulvar cancer in the age group 0-49 years for Australian women (1996-2001) and NT women by Indigenous status and district (1996-2005)
For VIN, the age-adjusted incidence rate for EA Indigenous women aged 0-49 years was 34.7 per 100,000 (95% CI 15.2–54.3), compared with 6.7 per 100,000 (95% CI 2.0–11.4) for Indigenous women living elsewhere in the Top End of the NT.
The incidence of vulvar cancer in NT women aged less than 50 years (Indigenous and non-Indigenous women combined) is the highest in the world (Figure 2).
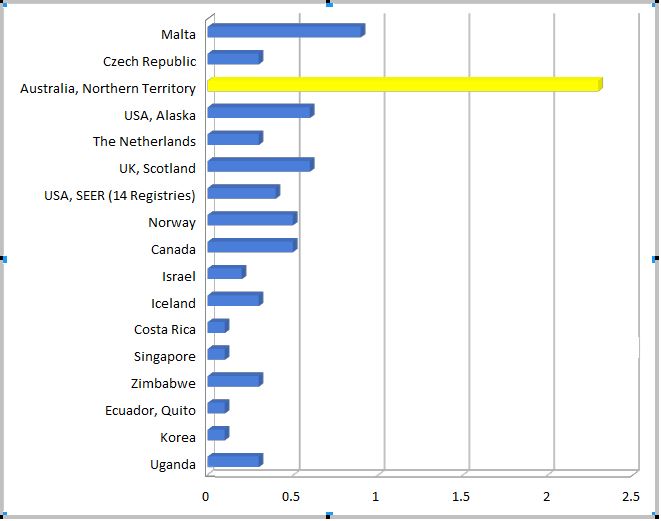
Figure 2 Incidence of vulvar cancer around the world in women aged less than 50 years
3. Human Papillomavirus Studies
Given that excess of vulvar cancer was restricted to younger women (aged less than 50 years), it was hypothesized the increased incidence of vulvar cancer in this population was the result of either:
- high prevalence of oncogenic genital HPV genotypes, or
- the presence of a highly virulent variant of HPV16 (the HPV genotype that causes most vulvar cancer).
A cross-sectional survey of 551 Indigenous women aged 18-60 years and living in EA found the prevalence of vulvar/vaginal/perianal high-risk HPV to be 39%, which is higher than that reported in previous studies.12 The prevalence of cervical high-risk HPV was 26%, which was similar to that reported by studies of other Australian women.
HPV16 was the most common type detected in both sites. There is some evidence to suggest that persistent infection of HPV16 at the vulva but not cervix may contribute to the excess. Investigation of this possibility in the EA population would require a long-term prospective longitudinal study, the feasibility of which has not been assessed.
Biopsies of vulvar cancer and VIN from women from EA and from other regions of the NT were genotyped for HPV.13 Biopsies from Arnhem Land were similar to those from other regions in the detection of any high-risk (HR) or possible HR and detection of HPV 16 specifically (Table 1). All HPV 16 variants detected were of the European prototype.
Table 1 Proportion of vulvar cancer and VIN biopsies with oncogenic HPV, NT 1996-2005
| Condition biopsied | Arnhem Land | Other regions | p-value |
| VIN | |||
| Any HR or possible HR HPV | 95% | 84% | 0.356 |
| HPV16 | 60% | 80% | 0.364 |
| Invasive cancer | |||
| Any HR or possible HR HPV | 100% | 80% | 0.473 |
| HPV16 | 70% | 70% | 1.000 |
These findings indicate that HPV is likely to be involved in the aetiology of disease in this population, but neither a higher prevalence of vulvar infection with HPV nor a particularly virulent strain of HPV explains the extent of the excess incidence of vulvar cancer in Indigenous women in EA.
It is expected that the vaccination of young people against HPV will contribute to a decline of vulvar cancer in this population, although there is insufficient data to quantify the expected decrease. The new nonavalent vaccine, currently being introduced, will likely increase the effectiveness of vaccination in relation to vulvar cancer, as it targets a wider range of HPV types.14 HPV vaccination has been available to women currently (in 2017) aged under 35, because vaccination was offered to women aged up to 26 years when the vaccine was introduced in 2007. Women currently aged over 35 have no vaccination protection.
4. Anthropological Study
A qualitative research study was undertaken through in-depth, open-ended interviews with purposively recruited participants including women who had had IVC or VIN, Aboriginal Health Workers, and doctors and nurses involved in caring for affected women. This study identified factors affecting diagnosis and treatment of women with vulvar cancer or VIN in EA communities, including: the private nature of the disease, the sense of shame associated with the condition, limited resources for health service delivery in EA, and a range of issues associated with the experience of relocation to metropolitan centres for treatment and specialist care.15,16 Interviewees were also asked about possible environmental exposures that might be contributing to vulvar cancer development, but no promising candidates for further investigation were identified.
5. Genetic Study
Gynaecologists from the Outreach Service reported that vulvar dysplasia appeared to cluster within particular family groups, suggestive of a genetic risk factor causing an inherited susceptibility, either to the oncogenic effects of HPV, or perhaps to another cause of vulvar cancer. Given the features of the disease in this population, it was hypothesised that any causative genetic variants were likely to be rare, to have a large effect size, and to stem from a mutation in a common ancestor.
A genetic case-control study of the vulvar cancer cluster was undertaken in 2011-2013, in which saliva samples were collected from 30 cases of vulvar cancer or VIN resident in the affected communities, and 62 unaffected controls, matched on age and community of residence.17 Genomic DNA was extracted from the samples, and genetic profiles generated by single nucleotide polymorphism genotyping using Illumina HumanOmni 2.5 BeadChips. Genome-wide association and identity-by-descent analyses, adjusted for relatedness within the sample, identified genomic regions of interest that are strongly suggestive of the involvement of a genetic risk factor.
More detailed genetic profiling was undertaken in 2014 using whole genome sequencing of 23 cases and 9 controls, and subsequent analyses have employed a variety of bioinformatics pipelines to prioritise variants of interest; this was a challenge given how little is known about normal genetic variation within this population. A group of four affected sisters was used as an initial filter, and then widened to include the whole sample. This approach identified a group of genetic variants in over 20 genes, the majority of which are involved in urogenital cancer pathways. These results indicate that, rather than one or two variants of large effect size, this cluster may be driven by the cumulative burden of rare functional variants.
Further whole genome sequencing is currently being undertaken in additional individuals to increase the sample size and allow a formal statistical ‘burden’ test to be completed prior to undertaking functional studies. The findings of the functional studies will assist in characterising the aetiology of this poorly understood malignancy.
6. Other Environmental Risk Factors
An increased prevalence of immunosuppressive conditions (such as diabetes, HTLV-1 or HIV) could also contribute to higher incidence of vulvar cancer.11 No case of HIV has ever been notified for an Indigenous person resident in the EA health district (pers. comm., P. Markey, 2017). Human T-Lymphotropic Virus-1 (HTLV-1) is endemic to Central Australia, but is virtually unknown in both East Arnhem and West Arnhem districts.18 Diabetes is more common in Indigenous Australians, particularly those living in remote communities; however, the prevalence of diabetes in EA has been estimated to be half that of Central Australia.19 Taken together, this suggests that immunosuppressive conditions are not the driving force behind this cancer cluster.
Women who participated in the genetic study were asked about their smoking status, and data relating to sexually transmitted infections and other HPV-related neoplasia (e.g. cervical, vaginal, perianal, anal or oropharyngeal) were extracted from their medical records.17 No association was found with any of these factors and diagnosis with VSCC or VIN except for previous diagnosis of cervical intraepithelial neoplasia (CIN). It is worth noting that the very high rates of smoking and STIs in this population may obfuscate any association between these factors and vulvar cancer. However, rates of smoking and STIs are also high in other remote Indigenous communities that do not experience a high incidence of vulvar cancer.
The anthropological study identified no promising candidate environmental exposures for further investigation as risk factors.
7. Current Epidemiology: Incidence and Recurrence
In recent years, the difference in vulvar cancer incidence between Indigenous and non-Indigenous Australians in the NT has been gradually diminishing.
Age-standardised rates of invasive vulvar cancer for the period 2006-2014 were 8.95 per 100, 000 [95% CI 5.26, 14.84] for Indigenous women and 1.02 [0.42, 2.32] in non-Indigenous women. The age-standardised rates of VIN for the same period were 5.45 per 100,000 [3.01, 10.01] in Indigenous women and 2.45 [1.51, 3.99] in non-Indigenous women.
In East Arnhem Indigenous women aged less than 50 (the group in which the original problem was identified), age-standardised rates of IVC have dropped to 13.17 [4.26, 31.28], compared to 3.99 [1.29, 9.53] for young Indigenous women in other Top End districts.
This apparent reduction in the gap in vulvar cancer incidence between East Arnhem and other Top End Indigenous women may be the result of increasing incidence of vulvar cancer worldwide (particularly HPV-dependent VSCC) in recent decades.2-4,20,21 Further, the increased attention to vulvar cancer prior to the first analysis may have led to increased ascertainment of cases in NT women outside Arnhem Land.
In Arnhem Land, however, we have evidence of a cluster of unusually aggressive HPV-dependent vulvar dysplasia. Indigenous women resident in Arnhem Land diagnosed with VSCC or VIN are more likely to experience a recurrence after treatment than non-Indigenous women or Indigenous women living elsewhere in the Northern Territory. They also experience less time before recurrence, and have higher mortality.
Indigenous women had 2.3-fold higher risk of recurrence compared with non-Indigenous women. The cumulative incidence of recurrence was higher for Indigenous than non-Indigenous women after five years (48.8% vs 30.2%) and ten years (64.1% vs 36.6%). At 5 years after diagnosis, Indigenous women had on average one recurrence per woman, and non-Indigenous women had 0.5.
The cumulative mortality for Indigenous women was 17.9% vs. 3.7% for non-Indigenous women after 5 years, rising to 19.5% vs. 19.6% after 10 years.
The differences in the natural history of HSIL (uVIN) and dVIN have resulted in different approaches to treatment. HPV-dependent HSIL has classically been known to develop slowly, occasionally spontaneously regress, and be less likely to progress to invasive cancer than HPV-independent dVIN.22 Thus, HPV-independent lesions have demanded immediate surgical treatment, whereas HPV-dependent uVIN could be treated with topical immune modulators (e.g. imiquimod) and monitored for longer periods before intervention. In this population, however, the unusually aggressive nature of the disease indicates that treatment needs to be prompt and thorough.
8. Disease Control Program
In collaboration with the Department of Health, the project team initiated a disease control program in 2006, focused on early detection and treatment. This comprised two main streams of activity: a community education program and a staff training program.
8.1 Community Education Program
Raising and maintaining awareness of the increased incidence of vulvar cancer in Indigenous women resident in the EA district has been a priority. The sense of shame associated with the disease and the limited access to healthcare in these remote communities may contribute to delays in seeking treatment. With no choice of doctor in many remote communities, women in Arnhem Land would, for instance, have difficulty talking to a male clinician about an issue deemed to be ‘women’s business’. To overcome these barriers, the community education program has focused on:
- Communicating the scale of the problem
- Providing information on signs and symptoms
- Recommending preventive action (HPV vaccination and regular Well Women’s Checks).
This has been effected through a combination of community based events and the development of resources to remain in the communities (Figure 3). Women’s cancer education events have been held periodically throughout the life of the project, often in conjunction with the Sexual Health Coordinator from the Centre for Disease Control Nhulunbuy, who offered Well Women’s Checks to attendees for opportunistic screening.
The resources for the communities have evolved over time: from an illustrated flipchart in English and Yolngu Matha; to an audio book with illustrations and recordings in English, two dialects of Yolngu Matha, and Anindilyakwa; to an animation in English and Yolngu Matha. While the first two iterations proved useful and culturally appropriate, the physical nature of the resources meant they were easily lost, particularly in clinics with high staff turnover. The animation will be hosted on the Menzies website, and will remain more democratically accessible to clinics, healthcare workers and community members on phones, tablets or computers.


Figure 3:Community education activities utilising vulvar cancer resources
8.2 Staff Training Program
Early detection and treatment relies on primary health care staff being aware of the need to look for signs of vulvar dysplasia, and how to identify it. Clinics in remote communities in the Northern Territory experience exceptionally high turnover of staff. Half of all nurses and Aboriginal health practitioners working in remote clinics leave within four months, 66% leave remote work altogether each year, and individual clinics can experience a 128% turnover of staff in 12 months.29 These retention patterns compromise health professionals’ awareness of the cluster and how to identify vulvar dysplasia. The staff training program aimed to:
- Provide clinical information on vulvar cancer and VIN, including how to recognise and diagnose it
- Encourage health professionals to include vulvar examination in cervical screening and antenatal care
These goals were achieved through:
- The development of a clinical training package, widely distributed on CD-ROM to relevant stakeholders
- Including vulvar cancer education in the orientation of remote practitioners and gynaecology staff
- Modifying the women’s health check protocol in the Women’s Business Manual
The high staff turnover means that these efforts need to be ongoing. We are in the process of updating the training tool, with the aim of increasing its utility in ongoing staff training efforts, and will likely take the form of a web-based application.
9. Investigators, Indigenous Reference Group and Partner Organisations
Stage 1
| Investigators | Associated Team Members |
| Prof John Condon | |
| Dr Alice Rumbold | |
| Prof Suzanne Garland | |
| Dr Jim Stankovich | |
| Dr Ngaire Brown |
Stage 2
| Investigators | Associated Team Members |
| Prof John Condon | Dr Rebekah McWhirter |
| Dr Pamela McGrath | Leonora Adidi |
| Dr Jim Stankovich | Elaine Lawurrpa Maypilama |
| Dr Briony Patterson | James Marthick |
| Dr Alice Rumbold | |
| Maria Nickels | |
| Dr Margaret Davy | |
| A/Prof Sepehr Tabrizi | |
| Dr Sarra Jamieson | |
| Debbie Taylor-Thomson |
Stage 3
| Investigators | Associated Team Members |
| Prof John Condon | Elaine Lawurrpa Maypilama |
| A/Prof Joanne Dickinson | Dr Matthew Field |
| Dr Rebekah McWhirter | Petr Otahal |
| Dr Alice Rumbold | James Marthick |
| Dr Russell Thomson | Bronwyn Carson |
| Dr Jacqueline Boyle | Dr Jac Charlesworth |
| A/Prof Sepehr Tabrizi | Dr Bennet McComish |
| Dr Peter Markey | |
| Debbie Taylor-Thomson |
Indigenous Reference Group Members (2007-2017)
| Sharon Munungarr | Daniella Dhamarrandji |
| Boyan Yunupingu | Elaine Lawurrpa Maypilama |
| Theresa Van Rangelrooy | Nikisha Dhurrkay |
| Sharon Wallace | Teresa Ngurruwuthun |
| Dipililnga Marika | Lisa Ngurruwuthun |
| Stephanie Yikaniwuy Dhamarrandji | Amanda Ngurruwuthun |
| Djanumbi Marika | Beth Djarrupi |
| Marlene Liddle | Alkira Wallace |
| Ritjilili Ganambarr | Mandy Ngalmilabak |
| Susan Ninikirri | Casey Daniels |
| Wanamula Gondarra | Helen Guypul |
| Banaka Lilpiyana | Marapalawuy Marika |
| Joyce Biyangu | Julie Gapalatharia |
| Judy Lirrirrinyin | Elaine Guymun |
| Stella Gondarra |
Sincere thanks go to our Indigenous Reference Group, the participating communities across East Arnhem and Groote Eylandt, and all the women who have participated in these studies.




Partner Institutions
Menzies School of Health Research
Menzies School of Health Research is Australia’s leader in Indigenous and tropical health research. Menzies works to better diagnose, prevent and treat disease and to partner with health services and communities to improve health outcomes. Menzies’ areas of expertise include Indigenous child and mental health, the social determinants of health, tropical and emerging infectious diseases, preventable chronic diseases, nutrition and international health.
Menzies Institute for Medical Research
Menzies exists to perform internationally significant medical research leading to healthier, longer and better lives for Tasmanians. We aim to advance human health and wellbeing by contributing significantly to knowledge on prevention and treatment of diseases including arthritis, cancer, multiple sclerosis, cardiovascular disease, diabetes type-2, osteoporosis, mental health, obesity and dementia.
National Health and Medical Research Council
NHMRC funding supports research across the full spectrum of health and medical research, from basic science through to clinical, public health and health services research. The research projects investigating the East Arnhem vulvar cancer cluster were funded by NHMRC project grant numbers 436013, 1003817 and 1060187.
Contacts
For further information, please contact:
Lead Investigator
Prof John Condon
E: John.Condon@menzies.edu.au
T: (03) 9819 2245
Project Manager
Dr Rebekah McWhirter
E: Rebekah.McWhirter@utas.edu.au
T: (03) 6226 4605
References
1. de Sanjosé S, Alemany L, Ordi J, et al. Worldwide human papillomavirus genotype attribution in over 2000 cases of intraepithelial and invasive lesions of the vulva. European Journal of Cancer 2013;49:3450-61.
2. Akhtar-Danesh N, Elit L, Lytwyn A. Trends in incidence and survival of women with invasive vulvar cancer in the United States and Canada: a population-based study. Gynecologic Oncology 2014;134:314-8.
3. Barlow EL, Kang Y-J, Hacker NF, Canfell K. Changing trends in vulvar cancer incidence and mortality rates in Australia since 1982. International Journal of Gynecological Cancer 2015;25:1683-9.
4. Meltzer-Gunnes CJ, Småstuen MC, Kristensen GB, Tropé CG, Lie AK, Vistad I. Vulvar carcinoma in Norway: A 50-year perspective on trends in incidence, treatment and survival. Gynecologic Oncology 2017:http://dx.doi.org/10.1016/j.ygyno.2017.03.008.
5. del Pino M, Rodriguez‐Carunchio L, Ordi J. Pathways of vulvar intraepithelial neoplasia and squamous cell carcinoma. Histopathology 2013;62:161-75.
6. Reyes MC, Cooper K. An update on vulvar intraepithelial neoplasia: terminology and a practical approach to diagnosis. Journal of Clinical Pathology 2014:jclinpath-2013-202117.
7. Bornstein J, Bogliatto F, Haefner HK, et al. The 2015 International Society for the Study of Vulvovaginal Disease (ISSVD) Terminology of Vulvar Squamous Intraepithelial Lesions. Journal of Lower Genital Tract Disease 2016;20:11-4.
8. Wills A, Obermair A. A review of complications associated with the surgical treatment of vulvar cancer. Gynecologic Oncology 2013;131:467-79.
9. Hinten F, Van den Einden L, Hendriks J, et al. Risk factors for short-and long-term complications after groin surgery in vulvar cancer. British Journal of Cancer 2011;105:1279-87.
10. Rottmann M, Beck T, Burges A, et al. Trends in surgery and outcomes of squamous cell vulvar cancer patients over a 16-year period (1998–2013): a population-based analysis. Journal of Cancer Research and Clinical Oncology 2016;142:1331-41.
11. Condon JR, Rumbold AR, Thorn JC, O’Brien MM, Davy MJ, Zardawi I. A cluster of vulvar cancer and vulvar intraepithelial neoplasia in young Australian Indigenous women. Cancer Causes & Control 2009;20:67-74.
12. Rumbold AR, Tan SE, Condon JR, et al. Investigating a cluster of vulvar cancer in young women: a cross-sectional study of genital human papillomavirus prevalence. BMC Infectious Diseases 2012;12:243.
13. Tan SE, Garland SM, Rumbold AR, et al. Investigating a cluster of vulvar cancers in young women: distribution of human papillomavirus and HPV-16 variants in vulvar dysplastic or neoplastic biopsies. Sexual Health 2013;10:18-25.
14. Joura EA, Giuliano AR, Iversen O-E, et al. A 9-valent HPV vaccine against infection and intraepithelial neoplasia in women. New England Journal of Medicine 2015;372:711-23.
15. McGrath P, Rawson N. The experience of relocation for specialist treatment for Indigenous women diagnosed with vulvar cancer in East Arnhem Land. Journal of Psychosocial Oncology 2013.
16. McGrath P, Rawson N. Key factors impacting on diagnosis and treatment for vulvar cancer for Indigenous women: findings from Australia. Supportive Care in Cancer 2013;21:2769-75.
17. McWhirter RE, Thomson RJ, Marthick JR, et al. Runs of homozygosity and a cluster of vulvar cancer in young Australian Aboriginal women. Gynecologic Oncology 2014;133:421-6.
18. Grivas R, Freeman K, Baird R. Human T-lymphotropic virus-1 serology in the Northern Territory: 2008-2011. Pathology 2014;46:644-8.
19. Zhao Y, Connors C, Wright J, Guthridge S, Bailie R. Estimating chronic disease prevalence among the remote Aboriginal population of the Northern Territory using multiple data sources. Australian and New Zealand journal of public health 2008;32:307-13.
20. Judson PL, Habermann EB, Baxter NN, Durham SB, Virnig BA. Trends in the incidence of invasive and in situ vulvar carcinoma. Obstetrics & Gynecology 2006;107:1018-22.
21. Lai J, Elleray R, Nordin A, et al. Vulval cancer incidence, mortality and survival in England: age‐related trends. BJOG: An International Journal of Obstetrics & Gynaecology 2014;121:728-38.
22. Halec G, Alemany L, Quiros B, et al. Biological relevance of human papillomaviruses in vulvar cancer.
23. Weberpals JI, Lo B, Duciaume MM, et al. Vulvar squamous cell carcinoma (VSCC) as two diseases: HPV status identifies distinct mutational profiles including oncogenic fibroblast growth factor receptor 3. Clinical Cancer Research 2017:doi: 10.1158/078-0432.CCR-16-3230.
24. Brotherton JML, Fridman M, May CL, Chappell G, Saville AM, Gertig DM. Early effect of the HPV vaccination programme on cervical abnormalities in Victoria, Australia: an ecological study. The Lancet 2011;377:2085-92.
25. Korostil IA, Ali H, Guy RJ, Donovan B, Law MG, Regan DG. Near elimination of genital warts in Australia predicted with extension of human papillomavirus vaccination to males. Sexually Transmitted Diseases 2013;40:833-5.
26. National HPV Vaccination Program Register. HPV Vaccination Coverage by Remoteness of area of residence for females aged 12-13 years in 2012. 2015:http://www.hpvregister.org.au/research/coverage-data/HPV-Vaccination-Coverage-Remoteness-2012.
27. Garland SM, Paavonen J, Jaisamrarn U, et al. Prior human papillomavirus‐16/18 AS04‐adjuvanted vaccination prevents recurrent high grade cervical intraepithelial neoplasia after definitive surgical therapy: Post‐hoc analysis from a randomized controlled trial. International Journal of Cancer 2016;139:2812-26.
28. Brotherton JML, Wrede CDH. Offering HPV vaccination to women treated for high-grade cervical intraepithelial neoplasia: what do you need to know? ANZJOG 2014;54:393-4.
29. Russell DJ, Zhao Y, Guthridge S, et al. Patterns of resident health workforce turnover and retention in remote communities of the Northern Territory of Australia, 2013-2015. Human Resources for Health 2017;15:52-63.

